Organization chart and team functioning
Paritarism as well as the representativeness of the different RTW stakeholders were the criteria used to define the structures (committees) and the operating mechanisms necessary for the development of the different sections of the website.
The organizational chart includes the following structures and the individuals involved have been identified in Appendix E (The Team):
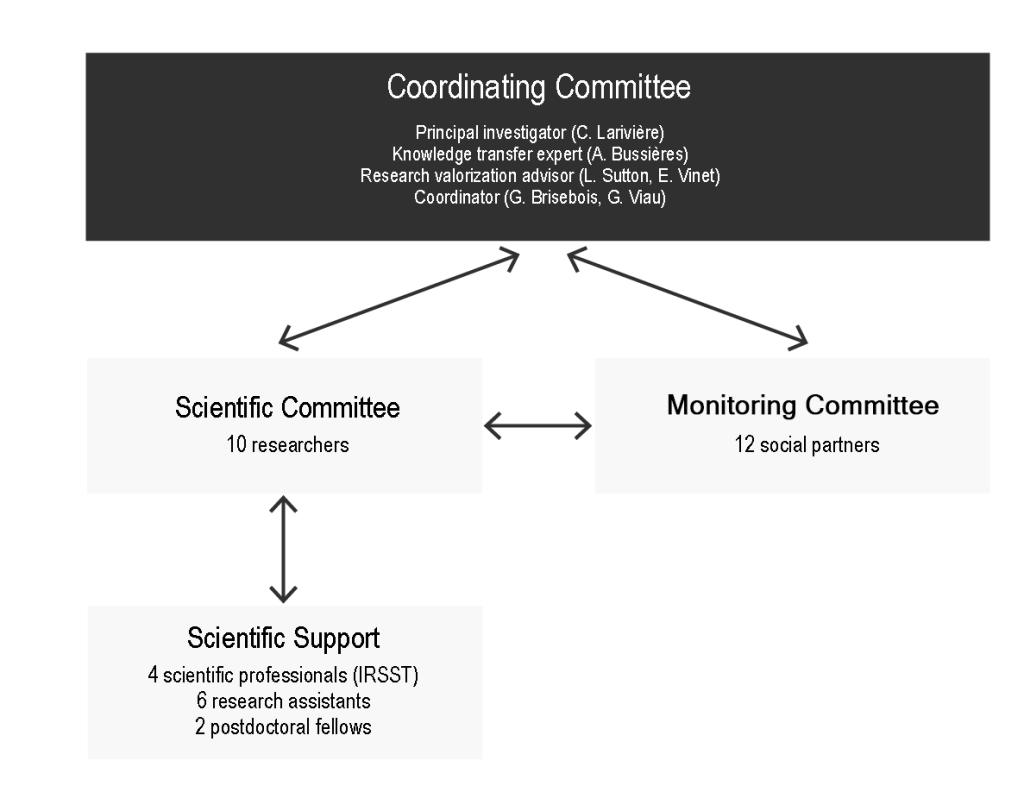
Coordination Committee (n = 4)
Responsibilities:
- Follow the work to make decisions of a more macroscopic nature.
- Organize and conduct meetings at appropriate times.
- Enforce objectives and timelines.
- Follow the scientific standards established for such a knowledge dissemination exercise.
The principal investigator, who recruited the members of the scientific committee, and the IRSST research valorization advisor, who recruited the members of the monitoring committee, served as interlocutors with these respective committees and co-facilitated the meetings. An expert in knowledge transfer acted as an observer and ensured that the procedures and decisions taken respected scientific standards. The IRSST’s occupational rehabilitation field coordinator organized the meetings (agenda management) and took the minutes of the meetings.
Scientific Committee (n = 10 researchers)
Responsibilities:
- Identify the themes.
- Participate in the development of content.
- Supervise research assistants.
This committee was mainly made up of senior researchers and other researchers with specific expertise, covering the different themes of the site.
Monitoring Committee (n = 12 social partners)
Responsibilities:
- Assist the Scientific Committee in making decisions on the content to be considered (advisory mandate).
- As representatives of the website’s intended users, provide feedback, comments and suggestions to ensure that the content posted online meets the needs of the intended users.
This committee was made up of three representatives from four categories of stakeholders (insurers, employers, union representatives, health professionals).
- The workers, although the main beneficiaries of the best practices described on this site, are not included in this committee. They were indirectly represented (union representatives).
- To ensure fair representation among the various stakeholders, only three representatives of health professionals were selected. These three representatives were deemed to cover the interventions related to the workplace. As mentioned earlier, interventions of an exclusively medical nature are not considered on this site. However, other health care providers are often involved in the RTW process (e.g., physiotherapists, psychologists or audiologists).
Scientific Support Committee (n = 6: 4 IRSST collaborators, 6 research assistants, 2 postdoctoral fellows)
Responsibilities:
- Identify and evaluate the quality of relevant scientific literature.
- Write and edit content based on feedback from other committees.
The IRSST’s permanent staff was involved in carrying out this research activity and in ensuring its sustainability. Members of the IRSST’s statistics monitoring team were involved in the section on the impacts of work disability, under the supervision of a researcher. The IRSST’s librarian was responsible for the documentary research for all sections of the site. The coordinator of the field of work rehabilitation supported the principal investigator in the creation of certain specific content.
Research assistants, under the direction of the researchers responsible for the content on worker assessment (modifiable factors of work disability and tools to measure them), effective interventions and best practices, also supported the scientific committee.
The postdoctoral fellows were involved in the worker assessment section, as the extraction and analysis of data from the literature search required very close monitoring of the research assistants involved in this section.
Operating and decision making mechanism
Five meetings of the Coordination Committee were held (November 1er 2016; December 5, 2016; October 5, 2017; April 30, 2018; and December 14, 2018) to establish a rigorous approach to the production and presentation of scientific content, for knowledge transfer purposes, and for the preparation of general meetings involving all committees.
Three general meetings, all held at the IRSST, were necessary:
- Meeting 1 (October 25, 2016): general information meeting to introduce themselves, present the research activity (objectives, modus operandi, etc.), answer questions, and launch activities;
- Meeting 2 (on March 1st 2017): validation of the needs and processes to be used for information gathering and content writing. A document outlining more detailed work plans, for each section, had been sent out a month before this meeting.
- Meeting 3 (end of project): summary presentation of the website sections with emphasis on what was added or removed during the work. Thanks to the team.
Two phases of consultation with the Monitoring Committee were carried out, in order to gather the comments of the social partners on the contents developed and sent to the Committee one month in advance:
- Consultation 1 (December 2017): four meetings, one for each stakeholder category (insurers, employers, unions or union representatives, health professionals), were conducted by three members of the coordinating committee. The content was then about 50% finalized. The revisions requested were moderately significant.
- Consultation 2 (late June-early July 2018): four meetings were required to consult with various members of the monitoring committee during this period, under the supervision of two members of the coordinating committee and a research assistant. The composition of the groups and the number of participants were varied. The content was approximately 95% complete. The revisions requested were relatively minor.
The coordinating committee was guided by existing recommendations for group composition and facilitation during this type of exercise (Kelson et al., 2012) and for managing conflicts of interest (Boyd et al., 2012).
In parallel, ten meetings of the Scientific Committee (or part of it) were held, together with members of the Scientific Support Committee, to deal with unforeseen events and threats to the scientific quality of the contents.
The possibility of bringing nuances to the contents developed was considered insofar as scientific rigor is not compromised and conflicts of interest do not alter the judgment of the participants. A form on the management of conflicts of interest was developed and used in this regard.
In an effort to allow the scientific content of each portion of content to be judged, the following criteria were used for the selection of information sources and their presentation, as well as to meet the more specific needs of the social partners:
- Prioritize sources of information based on the level of scientific evidence:
- Evidence-based (highest level of evidence) literature reviews and practice guidelines.
- Systematic literature review
- Other literature reviews
- Individual study (if sources 1, 2 and 3 are not available)
- Empirical data
- Proposals from the monitoring committee based on their needs, interests and values.
Thus, the content on the impacts of disability, the determinants of work disability, worker assessment and effective interventions is largely made up of sources 1, 2 and 3 (evidence), while the content on workplace practices is empirical data (source 5). Individual studies (source 4) were sometimes used. Finally, all of this was verified and commented on by the monitoring committee (source 6). Presentation of information in a way that clearly identifies content that is not evidence-based (when it does not exist), including proposals from the Monitoring Committee.
Bibliography
Boyd, E. A., Akl, E. A., Baumann, M., Curtis, J. R., Field, M. J., Jaeschke, R., . . . Schünemann, H. J. (2012). Guideline funding and conflicts of interest: Article 4 in integrating and coordinating efforts in COPD guideline development: An official ATS/ERS workshop report. Proceedings of the American Thoracic Society, 9(5), 234-242. doi: 10.1513/pats.201208-057ST
Kelson, M., Akl, E. A., Bastian, H., Cluzeau, F., Curtis, J. R., Guyatt, G., . . . Schünemann, H. J. (2012). Integrating values and consumer involvement in guidelines with the patient at the center: Article 8 in integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proceedings of the American Thoracic Society, 9(5), 262-268. doi: 10.1513/pats.201208-061ST